The molecular formula of aspirin is C 9 H 8 O 4. Carbon hydrogen and oxygen.
The Chemistry Of Aspirin The International Aspirin Foundation
C6H4O has appropriate mass not C4 BTW theres no problem at all with.
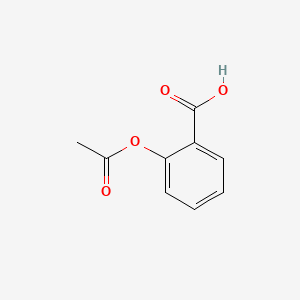
. The chemical formula for Acetylsalicylic Acid is C9H8O4. Graph showing the absorbance of the 11 dilution of the Aspirin solution used in the UV-Vis Spectroscopy with A 273 3602 and A 298 0737 Calculations. You can also see a benzene ring on the left side.
Into a second medium test tube place a match head-sized quantity of salicylic acid. The molecular geometry of aspirin is planar and its molecular mass is 180159 gmol. OH CH HC HC C3 Cl Br NOTE - Current drawing is incorrect as is.
These atoms are either single or double bonded together to form the overall structure of aspirin. The structural formula of aspirin or Acetylsalicylic acid is represented as- Aspirin is extensively used in the pharmaceutical sector. The spectrum showed a peak at 1717 cm -1 which indicated the ester carbonyl and a peak at 1687 cm -1 for the acid carbonyl.
As you can see- carboxylic acid and ester groups are present. Jul 14 2014. Up to 24 cash back The Lewis Structure of Aspirin Drawing of Aspirin Model of Aspirin Bibliography The Lewis Structure of Aspirin.
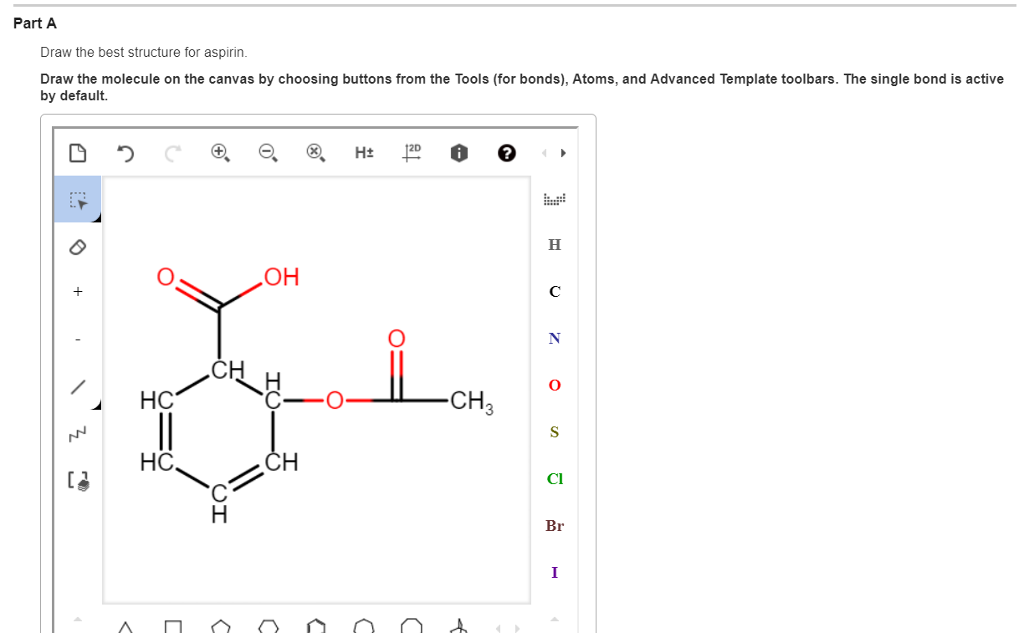
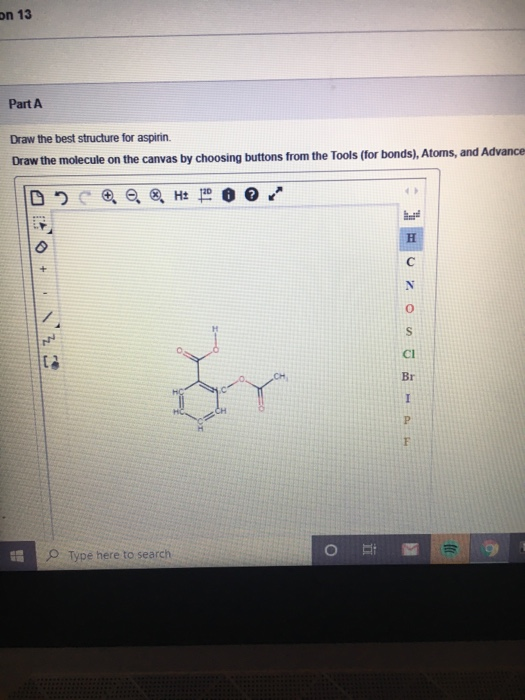
The Lewis Structure of Acetylsalicylic Acid Aspirin Nature of the Bonds. Part A Draw the best structure for aspirin. Aspirin C9H8O4 CID 2244 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
The third bond that occurs is H-Bonding. By looking at its chemical structure youll see that its composed of three different types of atoms. Hydrogen bonding is when the H of aspirin attracts to the N of NH3.
These peaks usually occur in the 1800-1715 cm -1 and 1725-1680 cm -1 range respectively. Add 5 mL of distilled water to the tubes containing the aspirin and the salicylic acid and to an empty tube. The most common way to prepare aspirin is by using Acid Anhydrides and by reacting acetic acid with salicylic acid.
The Molecule O OH HO O O acetyl salicylic acid aspirin. Its molecular weight is 180157 gramsmole. The remaining peak value from the synthesized aspirin is 167970 cm-1 which corresponds with values in both the ketone and carboxylic acid sections of table 24.
The temperature of an alcohol thermometer was equilibrated in a beaker of room temperature tap water. The mass spectrum is as follows. Similarly the extended formula for the same is said to be CH3COOC6H4COOH.
OH CH HC HC C3 Cl Br. M Aspirin M vial Aspirin M vial 2581 g 2311 g 260 g. Theoretical yield of Aspirin.
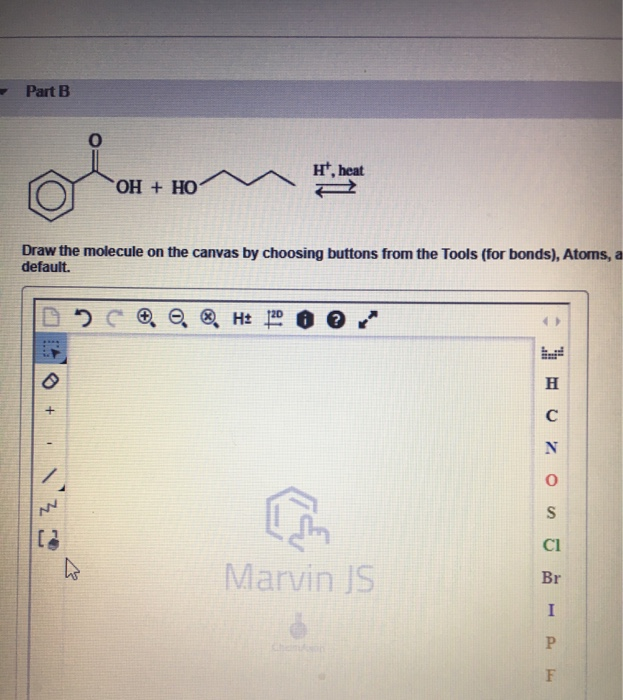
Draw the molecule on the canvas by choosing buttons from. Carboxylic acid is the. Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars.
Even though C X 6 H X 4 O is a molecular structure with mass 92 I dont think this will be formed since two bonds needs to be broken. Sodium acetate 020 g was added to one test tube. The structure of aspirin also indicates that there should be a peak for a carbonyl ester and a carbonyl acid.
This third tube will be used as a control for color comparison. Aspirin Formula and Structure. Absorbance spectra of Aspirin and Salicylic Acid.
By matching the synthesized aspirin peak at 174946 cm-1 to the peak values in table 24 it is evident that this peak represents the ester in the structure of aspirin. Part A Draw the best structure for aspirin. The second bond that occurs is dipole-dipole because they are both polar and the negative end of one molecule attracts to the negative end of the other.
Moderately Covalent O - 344 C - 255 344-255 089. Anti-inflammatory inhibition of the synthesis of prostaglandins. Cox-1Aspirin USAN also known as acetylsalicylic acid is a salicylate drug often used as ananalgesic to relieve minor aches and pains as an antipyretic to reduce fever and as an anti.
Aspirin is a salicylate drug often used as an analgesic to relieve minor aches and pains as an anti-inflammatory compound that inhibits Cox-1Target. Gastric irritation bleeding Apparition of new analgesics Tylenol Aspirin. This name along with its.
The active ingredient in aspirin and the chemical for which aspirin is the common name is acetylsalicylic acid. Also known as Aspirin acetylsalicylic acid ASA is a commonly used drug for the treatment of pain and fever due to various. Aspirin Synthesis Tap water was heated on a steam bath in a 250 mL beaker.
To determine the amount of aspirin acetylsalicylic acid in a sample the precise volume and concentration of the NaOH and the overall reaction must be known. The single bond is active by default. The NaOH serves as a secondary.
Place a match head-sized quantity of the pure aspirin into a medium test tube. Also the 92 peak is a major peak and the probability of two bonds breaking to lead to major peak is unlikely. Further the molecular mass of it is around 180159 g mol-1.
Moderately Covalent O - 344 H - 220 344-220 124 Oxygen and Carbon. The single bond is active by default. Lets go back and look at the chemical name of aspirin acetylsalicylic acid.
The chemical name of aspirin is acetylsalicylic acid. The molecular formula of aspirin is C 9 H 8 O 4. Up to 24 cash back The first bond that occurs is is dispersion because the two molecules are adjacent.
The molecule of the same forms by an aromatic ring having 2 functional groups in position orto. Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars. Salicylic acid 100 g 0005 g and acetic anhydride 20 mL 005 mL were added to each of four test tubes.
Solved On 13 Part A Draw The Best Structure For Aspirin Chegg Com
Solved Part A Draw The Best Structure For Aspirin Draw The Chegg Com

Aspirin Lewis Dot To Line Angle Structure Youtube
Solved On 13 Part A Draw The Best Structure For Aspirin Chegg Com

0 comments
Post a Comment